why metal carbonyls are called organometallics 12th metal carbonyl bonding and stability of complex compounds.
Metal carbonyls are fascinating compounds that have captured the interest of scientists for decades. These compounds are formed by the reaction of metal atoms with carbon monoxide, and they are characterized by unique structures, bonding, and properties that make them important in many areas of chemistry.
Classification and Types of Metal Carbonyls
Metal carbonyls can be classified based on their structure and the type of metal they contain. Some common types of metal carbonyls include:
- Mono- and polynuclear metal carbonyls
- Bridged metal carbonyls
- Terminal and bridging carbonyls
- Low- and high-spin metal carbonyls
The structures of these compounds can be very complex, and they often contain unique features such as metal-metal bonds, multiple bonding between metal atoms and carbon monoxide, and unusual coordination modes.
Structure and Bonding of Metal Carbonyls
The structure and bonding of metal carbonyls is complex, and it is still the subject of ongoing research. However, some important features include:
- Octahedral or tetrahedral coordination geometry around the metal atoms
- Multiple bonding between metal atoms and carbon monoxide
- Delocalized electrons in metal-metal bonds
These features are responsible for many of the unique properties of metal carbonyls, such as their ability to act as strong reducing agents, their tendency to form clusters and other complex structures, and their ability to interact with other molecules in unusual ways.
Uses of Metal Carbonyls
Metal carbonyls have numerous uses in chemistry, including:
- As catalysts in organic synthesis
- As precursors to metal nanoparticles
- As starting materials for the production of other metal complexes
They also have applications outside of chemistry, including:
- As metal plating and coating agents
- As lubricants and additives in engines and machinery
- As inhibitors for corrosion and other chemical reactions
 Metal Carbonyls in Organometallic Chemistry
Metal Carbonyls in Organometallic Chemistry
Metal carbonyls play a key role in organometallic chemistry, which is the study of organic molecules that contain metal atoms. The unique bonding and structural properties of metal carbonyls make them useful in a wide range of applications, including:
- As reactants in organic synthesis
- As reagents in catalysis reactions
- As starting materials for the production of other organometallic compounds
Overall, metal carbonyls are fascinating compounds that have made important contributions to many areas of chemistry. Their unique structures, bonding, and properties make them important in both academic research and industrial applications, and ongoing research into their properties is likely to lead to new discoveries and applications in the future.
 If you are searching about What’s An Organometallic? – Master Organic Chemistry you’ve came to the right page. We have 5 Images about What’s An Organometallic? – Master Organic Chemistry like What’s An Organometallic? – Master Organic Chemistry, Metal Carbonyls and also Metal Carbonyls|Organometallic Chemistry|Metal Carbonyls in. Read more:
If you are searching about What’s An Organometallic? – Master Organic Chemistry you’ve came to the right page. We have 5 Images about What’s An Organometallic? – Master Organic Chemistry like What’s An Organometallic? – Master Organic Chemistry, Metal Carbonyls and also Metal Carbonyls|Organometallic Chemistry|Metal Carbonyls in. Read more:
What’s An Organometallic? – Master Organic Chemistry
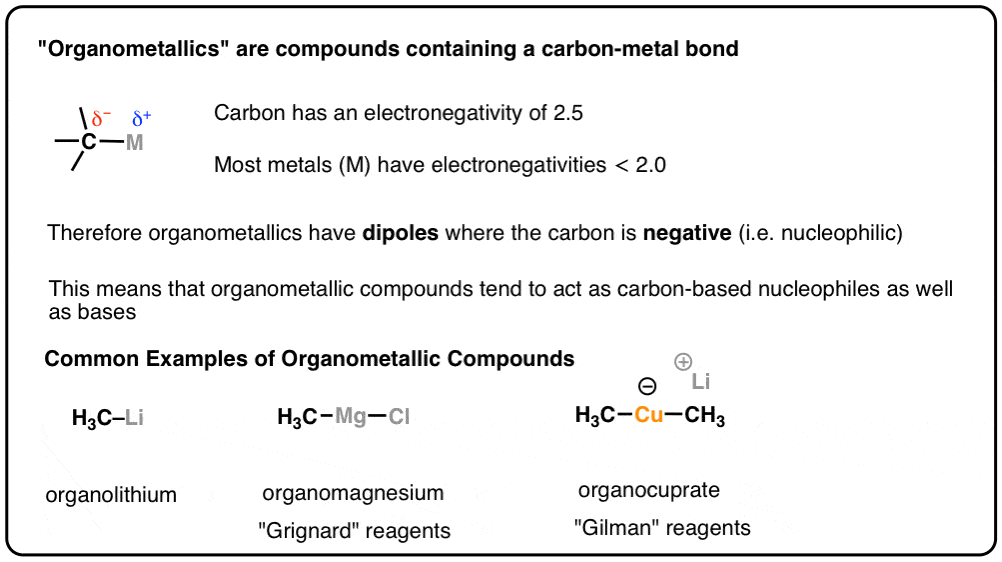 www.masterorganicchemistry.comorganometallic compounds metal carbon important bond electronegativity organic summary reactivity chemistry moc previously dipoles discussed ve
www.masterorganicchemistry.comorganometallic compounds metal carbon important bond electronegativity organic summary reactivity chemistry moc previously dipoles discussed ve
Metal Carbonyls
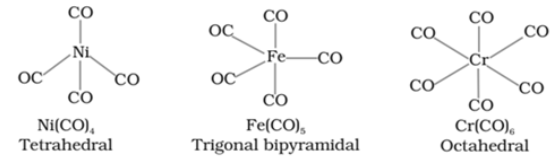 selfstudypoint.incarbonyls important bonding
selfstudypoint.incarbonyls important bonding
12th Metal Carbonyl Bonding And Stability Of Complex Compounds. - YouTube
 www.youtube.comMetal Carbonyls|Organometallic Chemistry|Metal Carbonyls In
www.youtube.comMetal Carbonyls|Organometallic Chemistry|Metal Carbonyls In
 www.youtube.comMetal Carbonyls: Classification, Types, Structure And Bonding, Uses
www.youtube.comMetal Carbonyls: Classification, Types, Structure And Bonding, Uses
 www.embibe.comcarbonyls classification
www.embibe.comcarbonyls classification
Metal carbonyls. Carbonyls classification. Metal carbonyls: classification, types, structure and bonding, uses